Important Safety Information
WARNING: Anaphylaxis
- Anaphylaxis has been reported after administration of KALBITOR.
- Because of the risk of anaphylaxis, KALBITOR should only be administered by a healthcare professional with appropriate medical support to manage anaphylaxis and HAE.
- Healthcare professionals should be aware of similarity of symptoms between hyper sensitivity reactions and HAE patients should be monitored closely.
- Do not administer KALBITOR to patients with known clinical hypersensitivity to KALBITOR.
CONTRAINDICATIONS
Do not administer KALBITOR to a patient who has known clinical hypersensitivity to KALBITOR
WARNINGS AND PRECAUTIONS: Hypersensitivity Reactions, Including Anaphylaxis
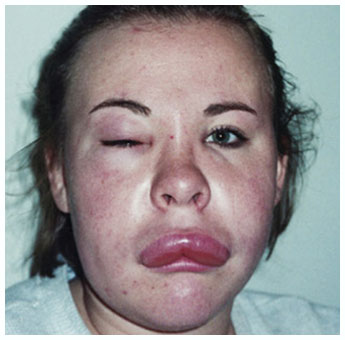
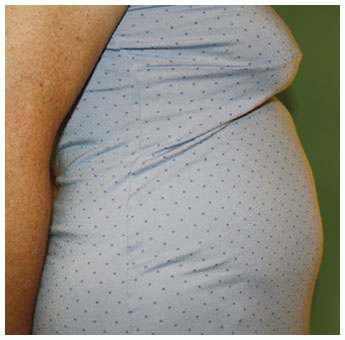
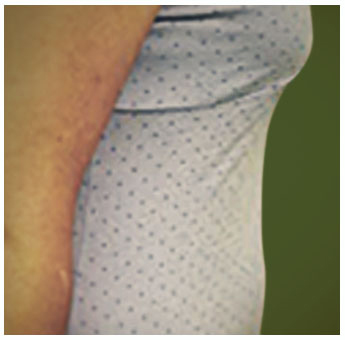
Potentially serious hypersensitivity reactions, including anaphylaxis, have occurred in patients treated with KALBITOR.
In 255 HAE patients treated with intravenous or subcutaneous KALBITOR in clinical trials, 10 patients (4%) experienced anaphylaxis. For the subgroup of 187 patients treated with subcutaneous KALBITOR, 5 patients (3%) experienced anaphylaxis. These reactions occurred within the first hour after dosing.
Symptoms associated with hypersensitivity reactions have included chest discomfort, flushing, pharyngeal edema, pruritus, rhinorrhea, sneezing, nasal congestion, throat irritation, urticaria, wheezing, and hypotension.
Other adverse reactions indicative of hypersensitivity reactions included the following: pruritus (5%), rash (3%), and urticaria (2%).
Patients should be observed for an appropriate period of time after administration of KALBITOR, taking into account the time to onset of anaphylaxis seen in clinical trials. Given the similarity in hypersensitivity symptoms and acute HAE symptoms, patients should be monitored closely in the event of a hypersensitivity reaction.
ADVERSE REACTIONS
The most common adverse reactions (≥3% and greater than placebo) in HAE patients were headache (8%), nausea (5%), diarrhea (4%), pyrexia (4%), injection site reactions (3%), and nasopharyngitis (3%).
There is a potential for immunogenicity with the use of KALBITOR. Overall, 20.2% of patients seroconverted to anti-ecallantide antibodies. Patients who seroconvert may be at a higher risk of a hypersensitivity reaction. The long-term effects of antibodies to KALBITOR are not known.
Please see the Full Prescribing Information, including Boxed Warning.