Hypersensitivity Reactions
WARNING: ANAPHYLAXIS
Anaphylaxis has been reported after administration of KALBITOR. Because of the risk of anaphylaxis, KALBITOR should only be administered by a healthcare professional with appropriate medical support to manage anaphylaxis and hereditary angioedema. Healthcare professionals should be aware of the similarity of symptoms between hypersensitivity reactions and hereditary angioedema and patients should be monitored closely. Do not administer KALBITOR to patients with known clinical hypersensitivity to KALBITOR. See Full Prescribing Information.
Drug Hypersensitivity in Clinical Trials
Symptoms associated with these reactions have included chest discomfort, flushing, pharyngeal edema, pruritus, rhinorrhea, sneezing, nasal congestion, throat irritation, urticaria, wheezing, and hypotension.1
- These reactions occurred within the first hour after dosing.1
- Other adverse reactions indicative of hypersensitivity reactions included the following: pruritus (5%), rash (3%), and urticaria (2%).1
- Patients should be observed for an appropriate period of time after administration of KALBITOR, taking into account the time to onset of anaphylaxis seen in clinical trials.1
Adverse Reactions
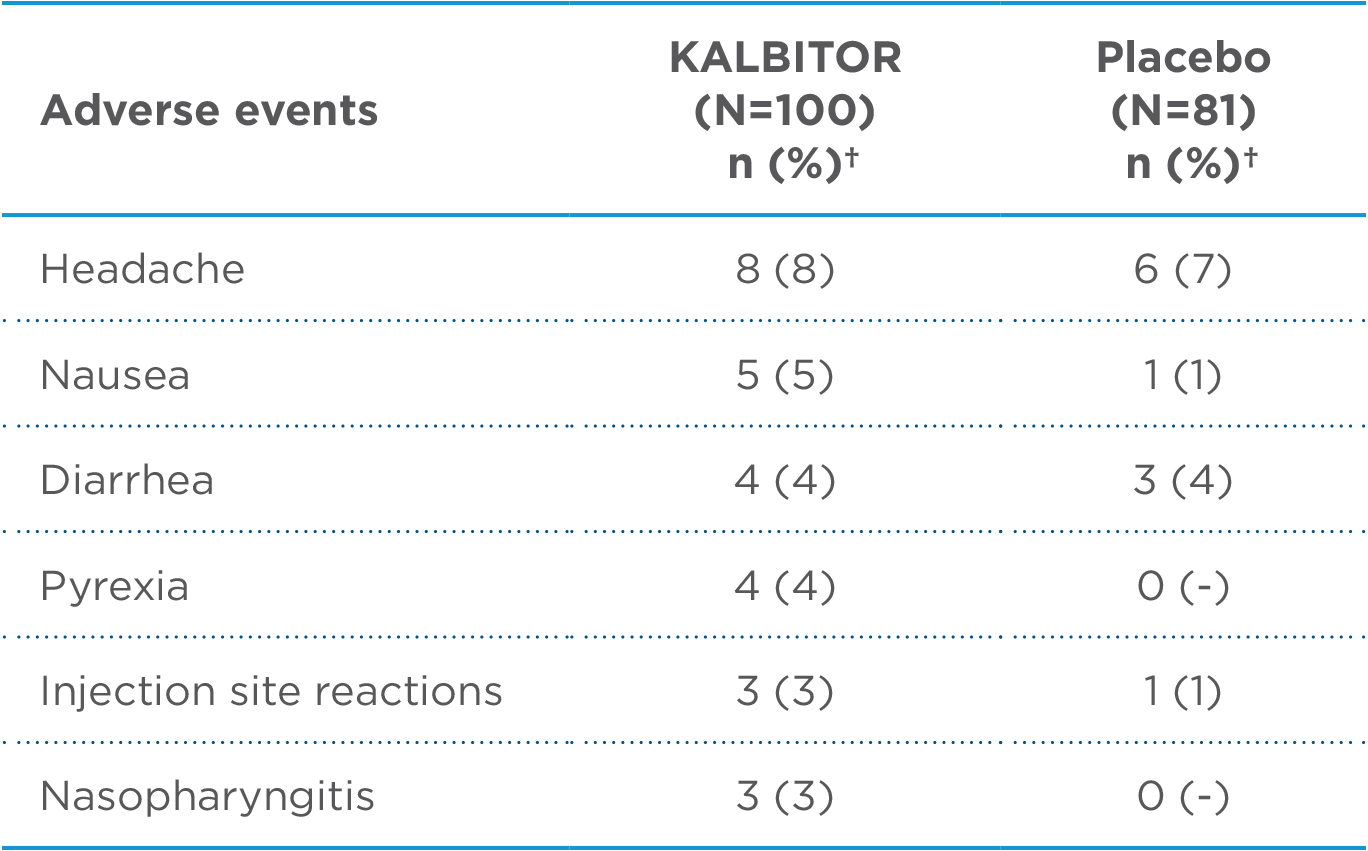
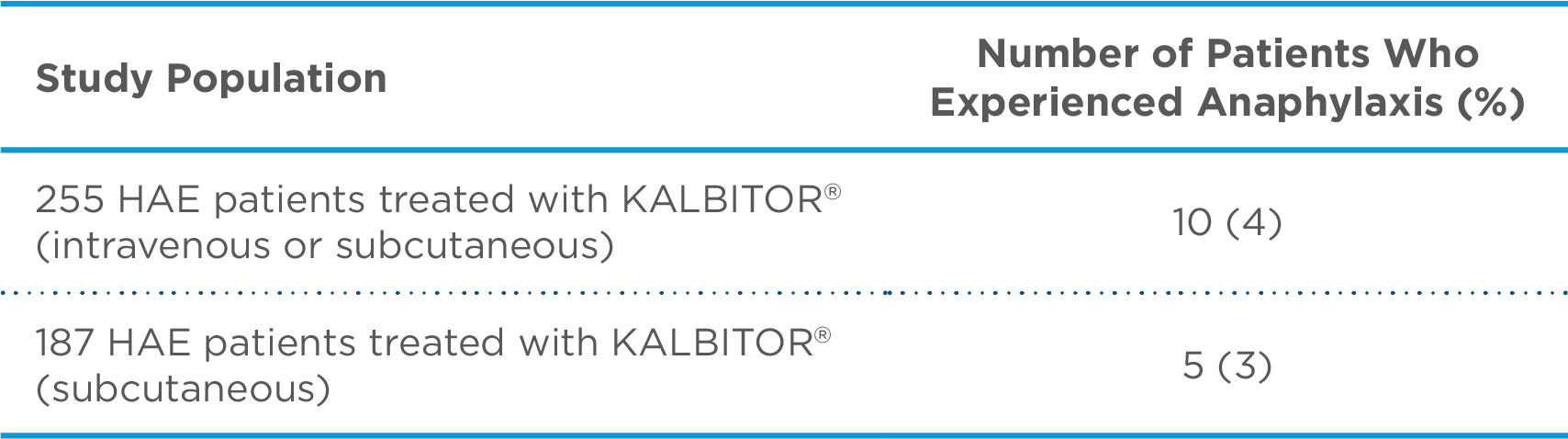
Overall, the most common adverse reactions in 255 patients with HAE were headache (16%), nausea (13%), fatigue (12%), diarrhea (11%), upper respiratory tract infection (8%), injection site reactions (7%), nasopharyngitis (6%), vomiting (6%), pruritus (5%), upper abdominal pain (5%), and pyrexia (5%). Anaphylaxis was reported in 4% of patients with HAE. Injection site reactions were characterized by local pruritus, erythema, pain, irritation, urticaria, and/or bruising. 1
Adverse Reactions Occurring at ≥3%, and Higher Than Placebo in KALBITOR-Treated Patients (30 mg Subcutaneous Dose)1*